Penny Vlahos a , Stephen L. Schensulb , Nishantha Nanayakkarac , Rohana Chandrajithd , Lalarukh Haiderb , Shuchi Anandf , Kalinga Tudor Silvae and Jean J. Schensul g
a Department of Marine Sciences, University of Connecticut, Groton, CT, USA;
b School of Medicine, University of Connecticut, Storrs, CT, USA;
c Faculty of Medicine, Kandy Teaching Hospital, University of Peradeniya, Kandy, Sri Lanka;
d Department of Geology, University of Peradeniya, Kandy, Sri Lanka;
e Department of Sociology, University of Peradeniya, Kandy, Sri Lanka;
f School of Medicine, Stanford University, Stanford, CA, USA;
g Institute for Community Research, Hartford, CT, USA.
ABSTRACT
Over the last two decades, a global epidemic of chronic kidney disease of unknown etiology (CKDu) has emerged in rural, arid, agricultural, lowland areas. Endemic regions have reported 15 to 20% prevalence among residents aged 30–60 years. CKDu is a progressive and irreversible disease resulting in renal failure and death in the absence of dialysis or a kidney transplant. While much of the research has focused on identifying etiology, this project seeks to ascertain factors associated with the rapidity of kidney disease progression in one of Sri Lanka’s CKDu endemic areas. A sample of 296 male and female residents aged 21 to 65 with moderate CKD, as measured by their serum creatinine level, and a clinical diagnosis of CKDu are followed using quarterly serum testing to track the rate of progression. A baseline survey administered to the entire sample addresses potential risk factors, supplemented by a short survey focusing on changes through time. Concurrently water, soil and air are tested at the local and household levels. The study is the first to foster a multidisciplinary approach that focuses on disease progression, identifying behavioural and exposure risk factors for rapid kidney function decline, in this progressively fatal disease.
ARTICLE HISTORY
Received 15 May 2018
Accepted 19 July 2018
KEYWORDS : CKDu; kidney disease; progression; agriculture; contaminants
Introduction
There is a rapidly expanding global prevalence of chronic kidney disease (CKD) among residents of low- and middle-income countries (LMICs) generated by the lifestyle changes contributing to the increase of diabetes and vascular disease. It is less well recognised that there is a parallel epidemic of chronic kidney disease of unknown etiology (CKDu) attributed to tubulointerstitial nephritis, that has emerged in Meso-America, South Asia (Gifford, Gifford, Eddleston, & Dhaun, 2017; Lunyera et al., 2016; Senevirathna et al., 2012) and other global regions in lowland, arid, agricultural communities. In these regions, the reported prevalence of CKDu reaches 15 to 20% among residents from 30 to 60 years of age with greater rates among men (Fischer et al., 2016, Torres et al., 2010).
Research efforts to date have focused on identifying the etiological factors of CKDu (Gifford et al., 2017). Hypothesised risk factors have included agrochemical chemical contamination of water, soil and air (Lebov et al., 2016; Rodríguez et al., 2006); dehydration resulting from intense work in hot and dry environments (García-Trabaninoa et al., 2015); behaviours such as heavy alcohol and tobacco use and agrochemical application (Hsu et al., 2011); infections such as leptospirosis and the hanta virus (Yang et al., 2017) and natural elements in soil and water such as cadmium, arsenic, and fluoride (Diyabalanage, Fonseka, Dasanayake, & Chandrajith, 2017). However, the great majority of publications investigating causes of CKDu are based on small sample sizes, powered (or sometimes underpowered) to explore single hypothesised causative agents. These studies suffer from significant residual confounding, the misdiagnosing of CKDu as indistinct from CKD, and the potential complexity and interaction of causative agents (Noble & Amerasinghe, 2014). To date, investigations of etiological factors have proved to be frustratingly inconclusive leaving health officials and endemic area residents with little guidance for prevention (WHO, 2016). This absence of a clear approach to amelioration leaves residents of endemic areas with no prevention guidelines, but with a high degree of concerns about the pathogenic risks of farming and water.
CKDu in Sri Lanka
In the early 1970s a donor-funded project implemented with the Government of Sri Lanka involved damming many of the country’s major rivers to create irrigation for the North Central and Eastern provinces in what had been heretofore arid and sparsely populated land. Landless rural peasants from the wet zone provinces were provided plots of land in these newly developed regions; with two growing seasons, these provinces became the rice bowl of Sri Lanka (Weeraratne & Wimalawamsa, 2015). Heavy production demands called for genetically-modified seed, application of increasing amounts of agrochemicals, extensive irrigation and mechanisation. The initial major health problem for people coming primarily from the wet to the dry zone areas was limited immunity for endemic malaria (in 2016, the WHO certified that Sri Lanka was malaria-free) (Silva, 2014). However, in the early 2000s the health care system began to notice a significant upsurge of chronic kidney disease without the precursor of diabetes that was disabling male and female heads of households. With the arrival of the new millennium, CKDu prevalence had risen to near 20% of residents in some of the endemic provinces, threatening the economic productivity of families and the country and resulting in excessive morbidity and mortality in the North Central and Eastern Provinces (Iqbal & Dissanayake, 2014).
There has been extensive research in Sri Lanka on the potential etiological factors that included an early white paper based on a collaborative WHO-Government of Sri Lanka study in 2012 (WHO, 2012). The first hypothesised culprit was thought to be agrochemicals that included pesticides, weedicides and fertilisers that leached into water, soil, air and food crops (Illeperuma, Dharmagunawardhane, & Herath, 2009). The early WHO study was flawed in its inability to distinguish CKDu from CKD. Other studies have identified natural substances in soil and water such as cadmium, arsenic, calcium, fluoride, selemnium, heavy metals and mycotoxin (Acquavella et al., 2003; Akerstrom & Sallsten, 2013; Athuraliya et al., 2011; Desalegn, Nanayakkara, Harada, Hitomi, & Chandrajith, 2011; Diyabalanage et al., 2017; Jayatilake, Mendis, Maheepala, & Mehta, 2013). Selenium deficiency was detected in certain areas (Iqbal & Dissanayake, 2014) however, results have been inconclusive; with no single common risk factor identified. Studies have identified both high and normal levels of these individual elements across both endemic/non-endemic areas and affected and non-affected individuals. Mycotoxins (aflatoxin and ochratoxin A) are known to cause tubulointerstitial disease in animals if ingested in large quantities mainly through contaminated feed (Fardos & Bokhari, 2010; Maaroufi et al., 1995). Mycotoxins have been detected in urine of both affected and unaffected individuals with CKDu. This suggests that exposure is ubiquitous in this area (Desalegn et al., 2011). Heat stress nephropathy has emerged as a strong potential etiology for Meso-American Nephropathy (Glaser et al., 2016) and has been proposed as well in Sri Lanka. To date, studies have been inconclusive (Jayatilake et al., 2013).
The kidney progression project (KiPP)
This study has taken a different tack, i.e. focusing on secondary prevention by seeking to identify the environmental, behavioural and clinical factors that contribute to variation in rates of progression from Stage 3 and early stage 4 CKDu (30–50% of kidney function). We designed a prospective cohort study of patients with an established diagnosis of CKDu to determine what factors might be associated with the rate of CKD progression. Although CKD and CKDu is a slow-moving disease, Sri Lankan nephrologists have reported considerable variation in the progression of the disease (Badrudeen et al., 2016; Fischer et al., 2017; Hettiarachchi et al., 2018). One possibility is that variation in exposure to a variety of factors could influence the rate of CKDu progression. Traditional case-control studies suffer from misdiagnosing CKDu, as controls could have early stage, undetected CKDu or CKD and ascertainment of remote exposures in cases may be subject to significant recall bias, thereby reducing the likelihood of obtaining reliable data. With the hypothesis that patients experiencing rapid decline in kidney function may be experiencing ongoing contact to causative or exacerbating agents, our approach has the capacity to directly measure relevant ongoing exposures. This study has the potential to explore the possibility of ongoing exposure to the etiological agents by analysing the relationship of various factors to the rate of progression. Secondly, we employ a multi-disciplinary approach. Our study casts a wide net to examine a series of demographic, familial, behavioural, occupational, clinical and environmental factors that contribute to the relative rapidity of disease progression. Since the biomedical science currently provides few approaches to prevent the disease or slow the rate of progression, we anticipate that this study will assist in identifying modifiable factors that could slow CKDu progression and allow individuals a longer and more productive life.
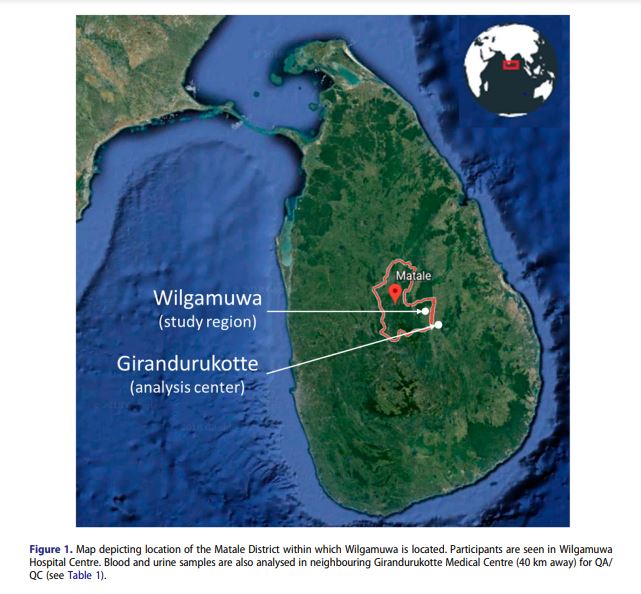
Study location
The CKDu endemic areas for KiPP is currently being conducted in the Wilgamuwa Divisional Secretariat of the Matale District (Figure 1). This region lays in the lowland, dry zone of the Central Province of Sri Lanka. The primary livelihood in the area is small-holder agriculture with rice farming, slash and burn cultivation of highland crops and manual wage labour including construction and sand mining from nearby rivers. Rice farming is partly irrigated and also depends on rainfall, which can be irregular and the application of chemical fertiliser and other agrochemicals. The total population in the Wilgamuwa Division in a 2012 population census was 29,494 consisting of 14,682 men and 14,812 women (17) and an estimated 8000 households with an average household size of 3.7. The entire Division is rural with no urban or plantation populations. Wilgamuwa is a socially and spatially marginalised area on the periphery of the Central Province; because of its remoteness and a high incidence of poverty; over 40% of the local population receives poverty alleviation benefits (Personal communication with the Assistant Director of Planning, Wilgamuwa Divisional Secretariat, February 22, 2017). In 2009, a blanket screening conducted by the Ministry of Health showed a high prevalence of CKDu (CKD data base, Ministry of Health, Central Province, Sri Lanka). A renal clinic was established to address the needs of residents. For those residents who had reached end-stage renal disease, dialysis and kidney transplantations are only available in Kandy, a roundtrip of six hours from Wilgamuwa.
Methods
Study design
KiPP is a prospective study of patients with identified moderate to advanced CKDu (estimated glomerular filtration rate [eGFR] of 20–59 ml min−1 1.73 m−2 ). This range corresponds to CKD Stage 3 and early Stage 4, selected so that there would be an opportunity to capture variation in progression with quarterly follow up over the project period. By design, the study focuses on the rate of progression from Stage 3 CKDu. As a result, the control group is derived from the CKDu cohort itself,
in which we evaluate the degree of exposures as they relate to differential, empirically-derived eGFR trajectories. We let the data define the number of sub-group patterns through ‘group-based trajectory modelling’ (Charnigo, Kryscio, Bardo, Lynam, & Zimmerman, 2011) in which empirically generated subgroups, following similar kidney function trajectories, are identified through best fit’ allowing us to examine the association of exposures to membership in these subgroups over 5 time points spanning Fall 2017 to May 2019) and evaluate the likelihood of covariates influencing the group membership and trajectory (Charnigo et al., 2011).
Originally, in August 2017 a blanket screening was conducted in the Wilgamuwa Secretariat by the Ministry of Health in collaboration with the KiPP. Urine dipstick and serum creatinine testing was conducted on all residents above 12 years of age. Individuals who were identified as having CKDu were visited and asked to consent participate in KiPP (See Figure 2). If they signed written consent, they were administered a comprehensive survey that included sociodemographic, behavioural and medical questions. Following survey administration, they were referred to the Wilgamuwa renal clinic for confirmation of the CKDu diagnosis including a physical exam, an ultrasound of the kidneys and a repeat of serum creatinine testing. Confirmation of CKDu resulted in a sample of n = 296.
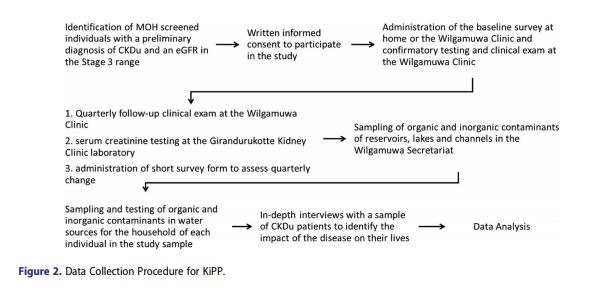
Participants are examined in Wilgamuwa Medical Centre (WMC) where blood draws and urine samples are collected, packed on ice and transported immediately to Girandurukotte Medical Centre (GMC) for analysis. Thus the samples undergo regular QA/QC at one common centre to avoid intercalibration concerns across laboratories. The field team resides in Wilgamuwa and performs all surveys and qualitative interviews in the participants’ homes. The team also partakes in clinical visits by ensuring participants are seen in a relatively efficient manner and oversees the transfer of all samples to the GMC which is 40 km from the WMC (the time lapse from collection to analysis is roughly 2 h).
Environmental sampling at macro and households are conducted which involved reservoirs used to irrigate rice fields and adjacent rivers at the macro level and all reported drinking water sources of each individual including household well water, any domestic reverse osmosis unit and rationed water delivered to the village by the district.
Patient identification and enrolment
The Ministry of Health personnel conducted a mass screening of Wilgamuwa residents that included blood serum creatinine and urine dipstick testing in August 2017. Using these data, we determined eligibility using the following criteria:
1) Age 21–65 years, 2) A repeat (after the Ministry of health screening) serum creatinine, with eGFR within 20–59 ml min−1 1.73 m−2 , 3) No diagnosis of diabetes (as evidenced by a recent fasting glucose test), 4) A urine dipstick negative for protein (i.e. < 1+), 5) Urine microscopy with less than 10 red blood cells per high power field, 6) A kidney ultrasound without many cysts, structural abnormalities or stones, 7) No hypertension or hypertension controlled on two or more medications. If the individual met the inclusion criteria, the study was described, and they were asked to sign a written consent form. If a patient had a biopsy-proven diagnosis of CKDu, these patients automatically entered the study as long as two serum creatinine measurements confirmed study-eligible eGFR range.
Clinical data
At the initial WHC visit following inclusion into the study, biometric data was collected and recorded, including age, sex, height (cm), weight (Kg) and body mass index (BMI) of the study participant. Blood pressure was checked and recorded by medical personnel using a manual sphygmomanometer and a stadiometer (height rod) was used to measure the height in meteres (cm) to
calculate BMI. Medical data such as past medical history, medications, previous laboratory data including renal ultrasound and renal biopsy (if available) were extracted from the medical records, held by the patient.
Each research assistant is responsible for 75 participants in different GN divisions. Quarterly follow up clinics are conducted every Monday and Saturday except public holidays. Roughly 35–40 patients are informed to participate on specific clinic days (usually 25–30 patients present in the clinic). All are registered as the first step. Weight, height and blood pressure are re-measured and participants are sent for repeat blood draws performed by a nurse in WHC. Two blood samples and Two Urine samples are taken for each participant. One blood sample and one urine sample are sent to the Lab in the WHC for full blood count (FBC) and urinalysis with microscopy respectively. The remaining blood samples (spun) and urine samples are transported to the Girandurukotte lab for lab analysis as outlined in Table 1. It takes up to 45 min to draw blood samples from all participants and an additional hour to transport blood and urine samples to Girandurukotte Lab from WHC. Samples are transported in a cool box with ice packs and blood samples are placed in a designated rack.
Laboratory data
Initial and quarterly laboratory investigations in the Girandurukotte lab include: serum creatinine (SCr), serum sodium (Na), serum potassium (K), serum bicarbonate (HCO3), serum calcium (Ca), serum phosphate, serum albumin (BCG), serum uric acid, hemoglobin (Hb) and hematocrit (Hct), total protein, total cholesterol, urine analysis, urine albumin to creatinine ratio. Serum Intact parathyroid hormone (iPTH) is not available in this remote clinic. If iPTH levels drawn at the central laboratory at Kandy Hospital are available in the participant’s personal record, it is entered in the data collection sheet.
SCr is calibrated to IDMS reference standards. SCr and serum uric acid are measured using an enzymatic assay in the Indiko analyser, (ThermoFisher Scientific). Na and K are measured using the ion selective electrode method and processed in the Human Plus machine. The end point reaction method using the Indiko machine is utilised for BCG, total protein, total cholesterol, Ca and P Urine albumin and creatinine analysed using the enzymatic method on the Humastar 600 machine. Instrument calibration is performed weekly.
Outcome variable
Chronic kidney disease is classified into 5 stages. The purpose of this classification is to help categorise the severity of CKDu, stratify for progression and complications and help guide management. The most common way to measure kidney function clinically is by serum creatinine level, which is a product of the breakdown of muscle with elimination via the kidneys. Baseline and quarterly serum creatinine testing to establish eGFR trajectory, identified as a change in eGFR, is determined over 18 months. Our primary outcome of interest in this study is the rate of progression. We will examine individual eGFR trajectories to empirically classify participants as experiencing rapid versus slow progression (Fan, 2009). In addition, as a secondary analysis, we will define rapid progression as any of: a decline of 30% or more in serum-creatinine based CKD-EPI estimated glomerular filtration rate (Coresh et al., 2014) or the need for renal replacement therapy (i.e. dialysis or transplant) over the study period.
Independent variables
We focused on three major domains: 1. Sociodemographic, familial, and behavioural, 2. clinical and laboratory, and 3. macro and micro environmental sampling (Table 1 details the data captured within each domain). At completion, each patient will have a detailed family history, and behavioural inventory covering their farming practices, substance use, psychological status, and health service utilisation. Any changes in clinical history (e.g. new diagnoses) including hospitalisations will be tracked during quarterly follow up. Each patient in the study sample has a set of values for levels of organic and inorganic contaminants in the water and soil in their immediate environments (Table 1).
Environmental protocol
Sampled participant drinking water sources including individual family wells, reverse osmosis water distributed by the township and bottled water. Water is analysed for inorganic ions, metals and agrochemicals listed in Table 1. The sampling period is designed to capture all periods of chemical application, field burning, and harvesting to understand seasonal variations of exposure. Inorganic water sampling and analysis is conducted by the Sri Lanka field team at the same sampling sites. Water is collected in acid washed containers for analysis of designated ionic species/heavy metals. Samples are analysed by an Ion Chromatograph and Inductively Coupled Plasma-Mass Spectrometer and selected duplicates are sent to the University of Connecticut, in order to QC the analysis. On-site measurements will be done for pH, conductivity (EC), and water temperature. All target agrochemicals and inorganic compounds will also be tested in rice consumed in the community, in locallymade alcohol, and in locally grown and chewed tobacco following the same methods outlined.
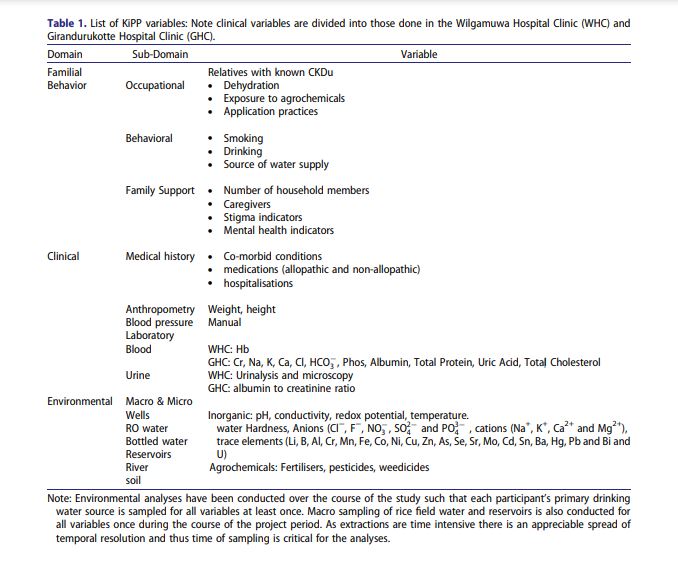
Water Hardness will be measured in all samples using the EDTA titration method. Anions (Cl−, F−, NO− 3 , SO2− 4 and PO3− 4 ) in samples will be measured with an Ion Chromatograph (Thermo Dionex ICS-1100). Na+ , K+ , Ca2+ and Mg2+ will be measured using atomic absorption spectrophotometry (on a Varian 240FS). All other trace elements (Li, B, Al, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Se, Sr, Mo, Cd, Sn, Ba, Hg, Pb, Bi and U) will be determined with a Thermo ICapQ Inductively Coupled Plasma Mass Spectrometer at the Department of Geology, University of Peradeniya. Instrumental calibrations will be performed using commercial standards and the instrumental internal drift will be corrected using 75Rh and 103Re internal standards. Certified standard samples of will be used as quality controlling standards.
For agrochemicals, one litre of water is extracted using solid phase extraction on a C-18 column. Cartridges are wrapped and mailed to the University of Connecticut for extraction and analysis. The cartridges are extracted using methods outlined in EPA Environmental Chemistry Methods (ECM) Index – E (2018), and analysed on an Agilent 5975C gas-chromatograph mass spectrometer (GCMS) using both electron Ionization (EI) and negative ionization (NCI) detectors.
Soil samples
Environmental Monitoring of Soil/Sediment samples involves a 5 square km grid system prepared using topographical maps for soil sample collection. Composite surface (up-to 25 cm) paddy/garden soil samples are collected and trace element analysis in soil samples are carried out using standard analytical methods. In this study, major and trace elements are measured. More attention will be given to As, Cr, Pb, Cd and U which are believed to have nephrotoxic effects. Approximately 100 samples will be analysed, sufficient to cover the microenvironments of the members of the study sample. Fertiliser samples (n = 30) are collected from markets in the study area and analysed for their trace metal contents giving particular attention toxic trace elements. Triple Super Phosphate (TSP) samples will also be collected randomly from the importers and analysed for their trace metal contents. Soil physical parameters such as pH, cation exchange capacity and total carbon will be determined. The soil (< 2 mm) samples will be finely ground with an agate mortar and then decomposed using microwave digester with mixed acid solutions, to determine the total element composition. The ICP-MS technique is the most appropriate instruments to obtain reliable trace element data (Chandrajith, Dissanayake, & Tobschall, 2005). International reference samples, reagent blanks and duplicate samples will be analysed for quality controlling purposes. Correlations of medical data and geochemistry with geographic incidence of CKDu will be conducted. Spatial distribution for trace elements in soils and water will be identified using GIS based mapping techniques. All sampling locations will be recorded using a high accuracy GPS and transferred directly to a data base. A database will be prepared using all collected data. ArcGIS or ArcView will be used for data processing purposes. Detail spatial distribution maps will be prepared using standard GIS.
Data management and analysis
All baseline and follow up survey instruments are entered into Redcap, a secure web application for building and managing online surveys and databases provided through the University of Connecticut. Data is analysed using both SPSS and SAS. We will use chi-square tests or 2- sample t-tests depending on the distribution of the predictor. After bivariate analyses, our ultimate goal will be to build a prediction model. Logistic regression can be used to regress the binary outcome of disease progression (‘rapid’ vs. ‘slow’) against a set of predictor variables (e.g. gets water from shallow well; elevated phosphate or glyphosate in drinking water or heavy tobacco use) and individual characteristics (e.g. age, gender) and community level variables (e.g. geographic area is high risk). Generalized linear models (GLM) will be utilised if we find progression to be normally distributed. Potential confounders will be considered for inclusion if, when added to the model, they change the parameter estimates of the major predictors in the model.
Power calculations
Sample size calculations converged on n = 300 to detect statistical significance and minimise type II errors. This is based on the estimation that of the n = 300 subjects with stage 3 and 4 CKDu enrolled, one-third will have more rapid disease progression, and two-thirds will have slower progression with expected outcomes for several predictors where we can detect odds ratios with at least 80% power.
Results
Sample characteristics
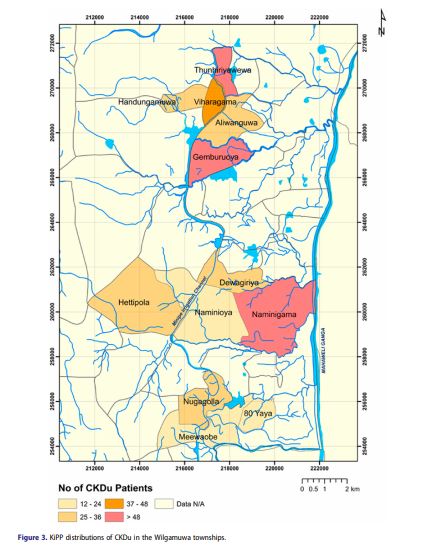
From August 2017 to February 2018, we approached 504 individuals whose Ministry of Health screening indicated an eligible CKD-Epi eGFRs. Of these 206 persons were ineligible, 1 declined to participate, and 5 could not be found at their listed home address. As of February 2018, 296 patients have consented and are enrolled in the study. Figure 3 is a GIS depiction of the Wilgamuwa region with preliminary distributions of CKDu prevalence.
The sample consists of 24% women (71 of 296) and 76% men (225 of 296). The average age is 53.6 ± 8.2 years (56.0 ± 7.4 and 52.9 ± 8.4 for women and men respectively). 51% of Participants have lived in Wilgamuwa all their lives. 88.5% of participants are married and living with spouse, 3.8% are separated or divorced, 6.1% are widowed, and 1.7% have never been married. 75.8% and 24.2 live in a nuclear or extended family respectively. Of the 296 participants 67% identify themselves as farmers and of these 75% have farmed for over 25 years. 63% of participants report frequent contact with agrochemicals. 16.1% of participants’ report household members also report having kidney disease. 10.7 report having a father with kidney disease and 10.4 report a mother with kidney disease both with a 45% mortality. 35% of participants report having at least one sibling with kidney diseases with 30% of those having died from the disease.
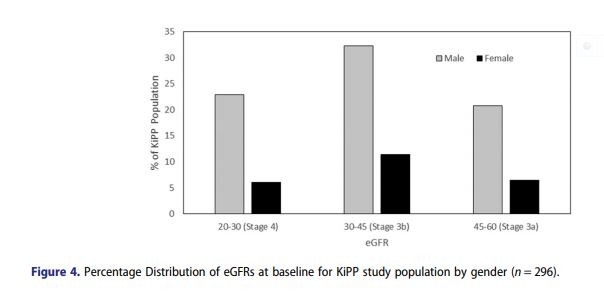
Figure 4 shows the eGFR ranges of the participant population which are relatively consistent across genders. At baseline 29% are CKDu Stage 3 and 71% of the participants are stage 4 CKDu respectively. Average serum creatinine values were 2.11 ± 0.58 (1.73 ± 0.49 and 2.23 ± 0.56 for female: males respectively) and eGFRs were 37.6 ± 11.5 (38.9 ± 11.9 and 37.3 ± 11.4 females: males respectively), (Figure 4. eGFRs distributions at baseline by gender).
Discussion
KiPP represents an ambitious attempt to undertake a rigorous, multi-disciplinary study investigating progression in CKDu, with multi-domain observations and measurements on patients living in remote, rural areas. During implementation of the protocol, we have found that participants are enthusiastic about the study and have a vested interest in slowing progression for themselves and affected family members. As such, there was relative ease in recruitment consent and follow up. We are also supported by an excellent data collection team with expertise in community outreach, research protocols, and clinical procedures.
Under the best of circumstances, field research combining environmental, behavioural and clinical data collection in remote rural areas of low- and middle-income countries presents challenges. However, in our study the greatest delays were administrative (e.g. IRB approval, fund transfers and institutional approvals).
There were anticipated and unanticipated challenges in the field. One major challenge is consistent communication with the participants as they are dispersed throughout Wilgamuwa, and the participants’ time is limited by their work schedule (especially during the harvest seasons). Extreme weather (heat and monsoons), local and national election season, rampaging elephants seeking rice in the paddies and venomous snakes, combined with inconsistent bus and auto rickshaw transport have created challenges for the field team. There was also a degree of research fatigue among the
respondents due to prior studies by various agencies triggered by increasing political significance of the disease for its impact on rural farming populations.
The choice of REDCap for data collection has been beneficial but made complicated by inconsistent internet coverage required for real time input. The team was required to collect data on paper and
enter into REDCap after the interview, doubling the work however this has been beneficial in tracing data entry errors. We are working on operationalising a mobile-based application that does not require real-time internet access to ease data collection and entry.
Follow-up visits are also difficult to facilitate due to the constraints on participants’ time and resources. In coordination with clinic medical officers, follow up clinic visits have been augmented as opportunities to receive refills in prescriptions, regular education sessions to educate on the importance of routine blood tests in CKDu management and that participants receive expedited care. Participants are contacted directly by the field team to arrange (1 week prior) and remind them (1 day prior) of appointments. New appointments are scheduled automatically for non-respondents. These measures improved follow-up compliance to well over 50%.
Laboratory capacity at the clinics is limited as well. The local Wilgamuwa hospital clinic was not able to receive higher volumes of patient serum or urine samples and the analyser was not IDMS calibrated, and thus we needed to arrange for transfer to a central laboratory where newer assays had to be purchased to allow for IDMS-calibration of serum creatinine.
CKDu has an as-yet unidentified pathology, and its largely silent nature makes direct exposure ascertainment difficult. A major advantage of the KiPP is the multiple disciplines represented among the PIs. We have three nephrologists (one in Sri Lanka and two in the US, three social scientists (2 in the US and one in Sri Lanka) and two biogeochemists (one in Sri Lanka and one in the US). Rosenfield argued for ‘..a transdisciplinary approach which can provide a systematic, comprehensive theoretical framework for the definition and analysis of the social, economic, political, environmental, and institutional factors influencing human health and well-being.’ This transdisciplinary approach goes beyond simply have each discipline doing ‘its thing’ but each participant sharing their perspectives and learning from each other to generate an integrated model that goes beyond disciplinary boundaries. When the work and the investigators are collaborating across cultures and countries, an additional dynamic of learning and sharing is introduced. Also, our study focus on the microenvironment and individual behaviour is unique and addressed the mosaic pattern of CKDu distribution.
Establishing causation across a number of environmental compounds and elements is a complex process, and in an environment where causation may be based on multi-stressors, attempts to isolate single causative factors may be difficult. This first-level screening in KiPP evaluates relative concentrations across all samples, assigns these concentrations to each participant and correlates them with participants’ kidney disease progression. In doing so we are looking for correlations and not causation. It is our hope that positive correlations will help guide future analyses that approach causation. Our primary aim is to contribute results which can reduce disease progression and thereby provide some benefit for the growing numbers of people with CKDu in Sri Lanka and in other LMICs. We will report results in Wilgamuwa, at the country level and internationally as soon as they are available.
Acknowledgements
First and foremost we thank the participants of the study. We are extremely grateful to the dedicated and enthusiastic team of young people H.P.M. Hewavitharane (research coordinator), S.M.R.G. Godavita (RA), H.P.N. Santhushya (RA), D.M.D.A. Dissanayake (RA), W.M.K.G.P. Wijerathne (RA), R.D.M.B. Karunrathne (RA), H.S. Sandaruwan (RA), A.M.C.M. Aththanayake (field assistant), P.N. Hewavitharane (field assistant), working in to collect participant and environmental data. This work was supported by the U.S. NIH Fogarty Institute under Grant R21TW010425. Dr Anand is supported by NIDDK K-23. The investigators would also like to acknowledge the support of the Sri Lankan Ministry of Health for facilitation of the above research.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by Fogarty International Center: [grant number R21TW010425]; National Institute of Diabetes and Digestive and Kidney Diseases: [grant number K-23].
References
Acquavella, J. F., Alexander, B. H., Mandel, J. S., Gustin, C., Baker, B., Chapman, P., & Bleeke, M. (2003). Glyphosate
biomonitoring for farmers and their families: Results from the farm family exposure study. Environmental Health
Perspectives, 112, 321–326.
Akerstrom, M., & Sallsten, G. (2013). Association between urinary excretion of cadmium and protein in a non-smoking population: Renal toxicity or normal physiology. Environmental Health Perspectives, 121(2), 187–191.
Athuraliya, N. T. C., Abeysekera, T. D. J., Amerasinghe, P. H., Kumarasiri, R., Bandara, P., Karunaratne, U., … Jones,
A. L. (2011). Uncertain etiologies of proteinuric-chronic kidney disease in rural Sri Lanka. Kidney International, 80
(11), 1212–1221.
Badrudeen, Z., Nanayakkara, N., Ratnatunga, N. V., Wazil, A. W., Abeysekera, T. D., Rajakrishna, P. N., … Alwis, A. P.
(2016). Chronic kidney disease of uncertain etiology in Sri Lanka is a possible sequel of interstitial nephritis. Clinical
Nephrology, 86(S1), 106–109.
Chandrajith, R., Dissanayake, C. B., & Tobschall, H. J. (2005). The abundances of rarer trace elements in paddy (rice)
soils of Sri Lanka. Chemosphere, 58(10), 1415–1420.
Charnigo, R., Kryscio, R., Bardo, M. T., Lynam, D., & Zimmerman, R. S. (2011). Joint modeling of longitudinal data in
multiple behavioural change. Evaluation & the Health Professions, 34(2), 181–200.
Coresh, J., Turin, T. C, Matsushita, K., Sang, Y., Ballew, S. H., Appel, L. J., … Levey, A. S. (2014). Decline in estimated
glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA, 311(24), 2518–2531.
Desalegn, B., Nanayakkara, S., Harada, K. H., Hitomi, T., & Chandrajith, R. (2011). Mycotoxin detection in urine
samples from patients with chronic kidney disease of uncertain etiology in Sri Lanka. Bulletin of Environmental
Contamination and Toxicology, 87, 6–10.
Diyabalanage, S., Fonseka, S., Dasanayake, D. M. S. N. B., & Chandrajith, R. (2017). Environmental exposures of trace
elements assessed using keratinized matrices from patients with chronic kidney diseases of uncertain etiology
(CKDu) in Sri Lanka. Journal of Trace Elements in Medicine and Biology, 39, 62–70.
EPA. (2018). Environmental Chemistry Methods (ECM) Index – E (2018). Retrieved from https://www.epa.gov/
pesticide-analytical-methods/environmental-chemistry-methods-ecm-index-e.
Fan, X. (2009). Power of latent growth modeling for detecting group differences in linear growth trajectory parameters.
Structural Equation Modelling, 10(2), 380–400.
12 P. VLAHOS ET AL.
Fardos, A., & Bokhari, M. (2010). Implications of fungal infections and mycotoxins in camel diseases in Suadi Arabia.
Saudi Journal of Biological Sciences, 17(1), 73–81.
Fischer, M. J., Hsu, J. Y., Lora, C. M., Ricardo, A. C., Anderson, A. H., Bassano, L., … Yaffe, K., (2016). CKD progression and mortality among hispanics and non-hispanics. Journal of the American Society of Nephrology, 27,
1–10.
Fischer, R. S. B., Mandayam, S., Chavarria, D., Vangala, C., Nolan, M. S., Garcia, L. L., … Murray, K. O. (2017). Clinical
evidence of acute Mesoamerican nephropathy. The American Journal of Tropical Medicine and Hygiene, 97(4),
1247–1256.
García-Trabaninoa, R., Jarquín, E., Wesseling, C., Johnson, R. J., González-Quiroze, M., Weiss, I., … Barregard, L.
(2015). Heat stress, dehydration, and kidney function in sugarcane cutters in El Salvador – A cross-shift study
of workers at risk of mesoamerican nephropathy. Environmental Research, 142, 746–755.
Gifford, F. J., Gifford, R., Eddleston, M., & Dhaun, N. (2017). Endemic nephropathy around the world. Kidney
International Reports, 2(2), 282–292.
Glaser, J., Lemery, J., Rajagopalan, B., Diaz, H. F., García-Trabanino, R., Taduri, G., … Johnson, R. J. (2016). Climate
change and the emergent epidemic of CKD from heat stress in rural communities: The case for heat stress nephropathy. Clinical Journal of the American Society of Nephrology, 11(8), 1472–1483.
Hettiarachchi, T. W., Sudeshika, T., Abeysundara, H., Yapa, R., Gunarathne, L., Hemage, R. D., … Nanayakkara, N.
(2018). Disease progression, mortality and morbidity of Chronic Kidney Disease of uncertain etiology (CKD-u),
Sri Lanka. BMC Nephrology, BNEP-D-17-00551 (submitted).
Hsu, Y.-H., Liu, W.-H., Chen, W., Kuo, Y.-C., Hsiao, C.-Y., Hung, P.-H., … Hsu, C.-C. (2011). Association of betel nut
chewing with chronic kidney disease: A retrospective 7-year study in Taiwannep. Nephrology, 16, 751–775.
Illeperuma, O. A., Dharmagunawardhane, H. A., & Herath, K. R. P. (2009). Dissolution of aluminium from substandard utensils under high fluoride stress: A possible risk factor for chronic renal failures in the North-Central province. Journal of the National Science Foundation of Sri Lanka, 37, 219–222.
Iqbal, M. C. M., & Dissanayake, C. B. (2014). CKDu in Sri Lanka. Science, 344(6187), 981.
Jayatilake, N., Mendis, S., Maheepala, P., & Mehta, F. R. (2013). Chronic kidney disease of uncertain aetiology:
Prevalence and causative factors in a developing country. BMC Nephrology, 14, 180–193.
Lebov, J. F., Engel, L. S., Richardson, D., Hogan, S. L., Hoppin, J. A., & Sandler, D. P. (2016). Pesticide use and risk of
end-stage renal disease among licensed pesticide applicators in the agricultural health study. Occupational and
Environmental Medicine, 73, 3–12.
Lunyera, J., Mohottige, D., Von Isenburg, M., Jeuland, M., Patel, U. D., & Stanifer, J. W. (2016). CKD of uncertain
etiology: A systematic review. Clinical Journal of the American Society of Nephrology, 11, 379–385.
Maaroufi, K., Achour, A., Betbeder, A. M., Hammami, M., Ellouz, F., Creppy, E. E., & Bacha, H. (1995). Foodstuffs and
human blood contamination by mycotoxin Ochrotoxin A: Correlation with chronic interstitial nephropathy in
Tunisia. Archives of Toxicology, 69, 552–558.
Noble, A., & Amerasinghe, P. (2014). Review of Literature on Chronic Kidney Disease of Unknown Etiology (CKDu) in
Sri Lanka. IWMI Working Paper 158. Colombo, Sri Lanka, International Water Management Institute (IWMI).
Rodríguez, T., Younglove, L., Lu, C., Funez, A., Weppner, S., Barr, D. B., & Fenske, R. A. (2006). Biological monitoring
of pesticide exposures among applicators and their children in Nicaragua. International Journal of Occupational
and Environmental Health, 12(2006), 312–320.
Senevirathna, L., Abeysekera, T., Nanayakkara, S., Chandrajith, R., Ratnatunga, N., Harada, K. H., … Koizumi, A.
(2012). Risk factors associated with disease progression and mortality in chronic kidney disease of uncertain etiology: A cohort study in Medawachchiya, Sri Lanka. Environmental Health and Preventive Medicine, 17(3), 191–198.
Silva, K. T. (2014). Decolonisation, development and disease: A social history of malaria in Sri Lanka. New Delhi: Orient
Blackswan.
Torres, C., Aragón, A., González, M., López, I., Jakobsson, K., Elinder, C., … Wesseling, C. (2010). Pathogenesis and
treatment of kidney disease decreased kidney function of unknown cause in Nicaragua: A community-based survey.
American Journal of Kidney Diseases, 55(3), 485–496.
Weeraratne, S., & Wimalawamsa, S. (2015). A major irrigation project (accelerated Mahaweli programme and the
chronic kidney disease of multifactorial origin in Sri Lanka. International Journal of Agricultural and
Environment Research, 1(6), 16–27.
WHO. (2012). WHO-Government of Sri Lanka study 2012.
WHO. (2016). WHO-International expert consultation on CKDu. Colombo, Sri Lanka: Author. Retrieved from http://
www.searo.who.int/srilanka/documents/report_international_expert_consultation_on_ckdu.pdf.
Yang, J., Sun, J. F., Wang, T. T., Guo, X. H., Wei, J. X., Jia, L. T., Yang, A. G. (2017). Targeted inhibition of hantavirus
replication and intracranial pathogenesis by a chimeric protein-delivered siRNA. Antiviral Research, 147, 107–115.
![]()


