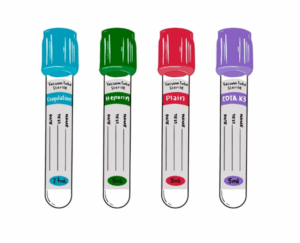
Accurate blood collection is an important step in patient care because most diagnoses depend on lab tests (WHO, 2010). Errors made before analysis, the “pre-analytical phase”, cause up to 70% of laboratory mistakes (Njoroge and Nichols, 2014; Dagher et al., 2019). Tubes used in venipuncture contain additives that directly influence the blood sample (ISO 6710, 2017). If the wrong order of tubes is followed, additives can contaminate later samples and change test results (CLSI, 2017; Šimundić et al., 2018).
The aim of this review is to explain the rationale behind blood collection tubes, the correct order of draw, key international guidelines, challenges in practice, and future innovations.
Blood collection tubes can be grouped in three ways:
- By additives – EDTA, heparin, citrate, fluoride/oxalate, and clot activators (CLSI, 2017; ISO 6710, 2017).
- By purpose – hematology (EDTA), coagulation (citrate), chemistry (serum or heparin plasma), serology, and molecular biology (Dagher et al., 2019).
- By color coding – tube caps follow international color standards, which help staff avoid mistakes (ISO 6710, 2017; Jacobsen et al., 2018).
Each blood collection tube contains a special chemical, called an additive, that helps preserve the sample for the right type of test. EDTA, found in lavender or purple top tubes, prevents clotting by binding calcium and also keeps blood cells in their natural shape, making it ideal for blood counts and blood films (CLSI, 2017; Jacobsen et al., 2018). Heparin, used in green top tubes, also prevents clotting by blocking thrombin and provides plasma for chemistry tests and some genetic studies, although it may interfere with PCR tests (Dagher et al., 2019). Citrate, in light blue top tubes, preserves clotting factors by binding calcium in a controlled ratio and is mainly used for coagulation tests such as PT and APTT (CLSI, 2017; WHO, 2010). Fluoride combined with oxalate, found in gray top tubes, stops glucose from breaking down in the sample, which is why it is useful for blood sugar testing (CLSI, 2017; WHO, 2010). Finally, serum tubes with clot activators or separating gel—red, gold, or tiger tops—speed up the clotting process and separate serum, making them suitable for chemistry and serology tests (CLSI, 2017; ISO 6710, 2017).
Several international guidelines provide direction for safe and accurate blood collection. The CLSI GP41 (2017) guideline defines venipuncture standards, including the correct order of draw, the number of tube inversions, and the proper filling of tubes. The World Health Organization (WHO, 2010) emphasizes safety, infection control, and best practices, especially in low-resource settings. ISO 6710 (2017) focuses on technical standards for blood collection tubes, including sterility, labeling, and closure systems. In addition, national and regional guidelines support consistency and patient safety worldwide (Šimundić et al., 2018).
The correct order of draw is very important to avoid contamination between tubes. According to the CLSI GP41 guideline (2017), blood should be collected in the following sequence: first blood culture bottles to keep them sterile, then light blue citrate tubes for coagulation studies, followed by red, gold, or tiger-top serum tubes for chemistry and serology. After that, green heparin tubes are used for plasma chemistry, then lavender or purple EDTA tubes for hematology, and finally gray fluoride/oxalate tubes for glucose testing. In capillary samples taken from a finger or heel, EDTA tubes are collected first to protect blood cell counts, and the other tubes are filled afterward (CLSI, 2017). This order is important because it prevents “carryover” of additives between tubes, which can give false results. For example, if EDTA contaminates another sample, it can lower calcium and increase potassium levels in the test results (CLSI, 2017; Jacobsen et al., 2018).
Table 1 – Order of Draw for Blood Collection Tubes with Additives and Common Uses (Based on CLSI GP41, 2017)
| Order | Color | Additive | Uses |
| 1 | Blood culture bottles | Nutrient broth (not shown in your original table) | Microbiology (bacteriology, sepsis diagnosis) |
| 2 | Light blue | Sodium citrate 3.2% | Coagulation studies (PT, PTT, INR) |
| 3 | Red (plain) | None | Chemistry panels (after serum separation) |
| 3 | Red (speckled) | Clot activator (silica particles) | Chemistry panels (after serum separation) |
| 3 | Gold | Clot activator (silica particles) & Gel separator | Chemistry panels (after serum separation) |
| 4 | Green (dark) | Sodium heparin | Chemistry panels (especially “stat” tests), Blood gas analysis |
| 4 | Green (light) | Lithium heparin (with or without gel) | Chemistry panels (especially “stat” tests), Blood gas analysis |
| 5 | Lavender | Potassium EDTA (K2EDTA) | CBC, Blood bank testing |
| 5 | Pink | Potassium EDTA (K2EDTA) | CBC, Blood bank testing |
| 6 | Gray | Sodium Fluoride & Potassium Oxalate / Sodium Oxalate | Blood glucose |
| — | Royal blue (no additive) | None | Trace element and heavy metal testing (less common) |
| — | Royal blue (with EDTA) | Potassium EDTA (K2EDTA) | Trace element and heavy metal testing (more common) |
Errors during collection can seriously affect results. If EDTA contaminates a sample, calcium can appear falsely low and potassium falsely high (CLSI, 2017; Jacobsen et al., 2018). If citrate tubes are underfilled, the blood-to-additive ratio changes, producing incorrect clotting times (WHO, 2010; CLSI, 2017). Heparin may interfere with PCR testing and cause false negative molecular results (Dagher et al., 2019). Glucose levels may also appear falsely low if blood is not collected in a fluoride tube (WHO, 2010). Delays in processing blood cultures reduce the chance of detecting infections (Venturelli et al., 2017).
Despite guidelines, challenges remain. Differences between tube manufacturers can affect results between laboratories (Dagher et al., 2019). Many healthcare workers lack full training in the correct order of draw (Šimundić et al., 2018). In low-resource settings, cost and limited access to proper tubes are common issues (WHO, 2010). Technical errors, such as hemolysis or clots caused by poor technique, also continue to affect sample quality (Njoroge and Nichols, 2014).
In conclusion, blood collection tubes are active tools designed to preserve samples in specific ways. Following the correct order of draw is essential to avoid contamination and false results. International guidelines from CLSI, WHO, and ISO provide clear standards to improve patient safety and reliability of laboratory testing. Ongoing training for healthcare workers and the use of newer tube technologies can further reduce errors and strengthen laboratory quality.
References:
- CLSI (2017) GP41—Collection of Diagnostic Venous Blood Specimens. Wayne, PA: Clinical and Laboratory Standards Institute.
- WHO (2010) WHO guidelines on drawing blood: best practices in phlebotomy. Geneva: World Health Organization.
- ISO 6710 (2017) Single-use containers for venous blood specimen collection – Requirements and test methods. Geneva: International Organization for Standardization.
- Dagher, G. et al. (2019) ‘Pre-analytical processes in medical diagnostics: new regulatory requirements and standards’, New Biotechnology, 52, pp. 121–128.
- Jacobsen, K.K. et al. (2018) ‘Order of draw practices in venous blood sampling’, Clinical Biochemistry, 56, pp. 113–117.
- Njoroge, S.W. and Nichols, J.H. (2014) ‘Risk management in the clinical laboratory’, Annals of Laboratory Medicine, 34(4), pp. 274–278.
- Šimundić, A.-M. et al. (2018) ‘Joint EFLM-COLABIOCLI recommendation for venous blood sampling’, Clinical Chemistry and Laboratory Medicine, 56(12), pp. 2015–2038.
- Fabre, V., Carroll, K.C. and Cosgrove, S.E. (2021) ‘Blood culture utilization in the hospital setting: a call for diagnostic stewardship’, Journal of Clinical Microbiology, 60(3), e01005-21.
- Venturelli, C. et al. (2017) ‘Impact of pre-analytical time on the recovery of pathogens from blood cultures’, PLoS ONE, 12(1), e0169466.